Abstract
Introduction: Anthracycline-based combination chemotherapy is a standard first-line treatment for anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL) both in adults and children, which results in favorable clinical outcomes. However, a standard therapy has not been established for recurrent or refractory diseases. Because many treatment options, such as hematopoietic stem cell transplantation, are available, patients with diseases that are resistant to conventional chemotherapies have particularly poor prognosis. Brentuximab vedotin, an anti-CD30 antibody drug conjugate, has improved the prognosis of recurrent or refractory ALCL; however, other types of novel agents have not yet been investigated. ALK inhibition could be a promising therapeutic strategy for ALK-positive malignancies. Therefore, this study aimed to determine the efficacy and safety of alectinib, a second-generation ALK inhibitor, in patients with recurrent or refractory ALK-positive ALCL.
Methods: In this open-label, phase II trial, patients with recurrent or refractory ALK-positive ALCL diagnosed by histological examination were eligible. Other major inclusion criteria were as follows: age >6 years, ECOG performance status 0-2, at least one measurable lesion, and preserved organ functions. Alectinib 300 mg was administered orally twice a day (600 mg/day). Patients who weighed <35 kg were administered reduced doses of alectinib, i.e., 150 mg twice a day (300 mg/day). The primary endpoint was the response rate according to the Revised Response Criteria for Malignant Lymphoma. The secondary endpoints were pharmacokinetics, safety in children, complete response rate, response duration, progression-free survival, event-free survival, overall survival, and adverse events (AEs). All patients who received at least one dose of alectinib were evaluated for responses and toxicities. We expected that a response rate of 85% and a statistical power of 79% could be obtained for 10 patients with an alpha level of 0.05 (one tailed). This study is registered in UMIN-CTR (UMIN 000016991).
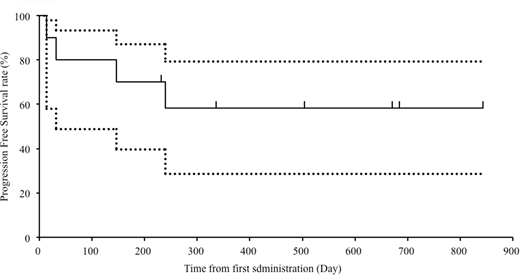
Results: Ten patients were enrolled in the study from May 2015 to November 2017. The median age was 19.5 (range, 6-70) years. Of 10 patients, 8 were administered alectinib 600 mg/day and 2 were administered with 300 mg/day. Objective tumor responses were documented in 8 of 10 patients (80%; 90% confidence interval: 56.2-95.9), with CR in 6 and PR in 2 according to the independent review committee. Two patients underwent stem cell transplantation after alectinib treatment. Five patients continued to take alectinib at the data cut-off. One-year overall survival and progression-free survival rates were 70.0% and 58.3% (Fig), respectively. Three patients died owing to disease progression. A severe AE was observed in 1 patient (grade 3 acute pharyngitis). Grade 4 AEs were observed in 1 patient (neutropenia). Common AEs observed were diarrhea in 2 patients, upper respiratory infection in 3, elevated alkaline phosphatase in 3, and maculopapular rash in 4. No unexpected AEs were experienced. The mean steady state peak concentration of alectinib was 479.5, 676.7, and 391.1 ng/mL in patients aged <15 years administered 300 mg/day, those aged <15 years admisnitered 600 mg/day, and those aged >15 years administered 600 mg/day, respectively. The mean time to reach the peak concentrations was 4, 4, and 5 h, respectively.
Conclusion: Alectinib showed favorable clinical activity and was well tolerated in patients with ALK-positive ALCL who progressed on standard chemotherapy. This study demonstrated similar pharmacokinetic profiles between pediatric and adult patients. Therefore, alectinib could be a suitable treatment for both pediatric and adult patients with recurrent or refractory ALK-positive ALCL.
Sekimizu:Eli Lilly and Company: Speakers Bureau. Kada:Bayer Yakuhin, Ltd: Membership on an entity's Board of Directors or advisory committees. Nagai:HUYA Bioscience International: Research Funding; Bayer Yakuhin Ltd.: Research Funding; Esai Co., Ltd.: Honoraria, Research Funding; Ono Pharmaceutical Co., Ltd.: Honoraria, Research Funding; AstraZeneca plc.: Research Funding; Otsuka Pharmaceutical Co., Ltd.: Research Funding; Janssen Pharmaceutical K.K.: Honoraria, Research Funding; Kyowa Hakko Kirin Co., Ltd.: Honoraria, Research Funding; Roche Ltd.: Honoraria; SymBio Pharmaceuticals Limited: Research Funding; Celgene Corporation: Honoraria, Research Funding; Sanofi K. K.: Honoraria; Mundipharma K.K.: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Takeda Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Gilead Sciences Inc.: Honoraria, Research Funding; Solasia Pharma K.K.: Research Funding; Zenyaku Kogyo Co., Ltd.: Honoraria, Research Funding; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Abbvie G. K.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal